|
TRANSLATE THIS ARTICLE
Integral World: Exploring Theories of Everything
An independent forum for a critical discussion of the integral philosophy of Ken Wilber
 Andrew P. Smith, who has a background in molecular biology, neuroscience and pharmacology, is author of e-books Worlds within Worlds and the novel Noosphere II, which are both available online. He has recently self-published "The Dimensions of Experience: A Natural History of Consciousness" (Xlibris, 2008). Andrew P. Smith, who has a background in molecular biology, neuroscience and pharmacology, is author of e-books Worlds within Worlds and the novel Noosphere II, which are both available online. He has recently self-published "The Dimensions of Experience: A Natural History of Consciousness" (Xlibris, 2008). SEE MORE ESSAYS WRITTEN BY ANDY SMITH
Studies of masks show beyond a shadow of doubt that they are very effective at blocking a large proportion of air-borne particles of the size that carry viruses from an infected person.
When Scientists DissentThe Minority View of the PandemicPart 2: The Effectiveness of FacemasksAndy Smith
The physical evidence is overwhelming that masks provide an effective barrier to viruses.
In Part 1 of this series, I addressed the evidence for the effectiveness of lockdowns. Given the enormous economic and psychological effects of forcing businesses to close and people to stay home, it's entirely understandable that many would resist these restrictive measures, and question whether their benefits outweigh their costs. The same cannot be said of wearing facemasks, which are a minor inconvenience, certainly far more preferable than sheltering at home or not working, to the extent that choice is possible. Yet in many countries, and particularly in the U.S., there is enormous popular resistance to wearing facemasks. Large numbers of people see them as not only ineffective, but imposed intentionally by the government as a plan to extend control over the citizenry. Deaf to the pleas of mask advocates to think about the safety of others, opponents of masks have not only ridiculed those who wear them, but marched in protest, caused major disturbances when barred from entering certain businesses that require masks, and even, literally, killed people over the issue. The opposition to masks has been aided and abetted by scientists, and others who appeal to science, who claim that there's no evidence that masks reduce transmission of the virus—that in fact, there is not only absence of evidence, but evidence of absence. To begin, I note that there are two kinds of evidence bearing on this issue: physical and epidemiological. The physical evidence, obtained from numerous studies measuring the ability of masks to block the virus-containing droplets and aerosols that we exhale when coughing, talking or just breathing, is compelling. While masks don't block 100% of virus particles, many types of masks can block a very high proportion of these particles, of varying sizes. The epidemiological evidence, which examines whether large numbers of people wearing masks have an effect on the rate of positive cases, is less clear-cut, but still consistent with, and supportive of, the physical evidence. The particulars of particulates: the physical evidence for masksWhen size doesn't matterMany studies have shown that masks block exhalation of virus—not simply the N95 masks that are specifically designed to filter out viral particles, but surgical masks used to protect against bacteria, and even home-made cloth masks (van der Sande et al. 2008; Lai et al. 2012; Noti et al. 2012; Milton et al. 2013; Zangmeister et al 2020; Lustig et al 2020; Ma et al 2020; Konda et al. 2020; Bartosko et al. 2020). A single coronavirus has a diameter of about 125 nm (nanometer, one-millionth of a millimeter), so it might seem surprising that masks made of relatively crude and porous fabrics could block emission of a virus. In fact, even N95 masks feature pore sizes, 300-500 nm, that are larger than a single virus, leading some mask opponents to argue that they must be ineffective (Rancourt 2020). Such opponents also cite studies that show that even N95 masks, as well as surgical masks and cloth masks, may not provide as much protection as generally thought (Balazy et al. 2006; Lee et al. 2008; Rengasamy et al. 2010). However, this view betrays several misunderstandings about viruses and masks. First, naked viral particles are not exhaled by infectious individuals. The newly synthesized viruses become suspended in bodily fluids, such as saliva, and it's these fluids that, upon speaking, coughing or just breathing, are expelled into the air in the form of aerosols or droplets. These particles range greatly in size, from less than 1 µ (micron, one thousand times as great as a nanometer, and one thousand times smaller than a millimeter) to 100 µ or more in diameter. Generally speaking, particles 5 µ or less in diameter are classified as aerosols, while larger particles are considered droplets. Aerosols, because of their small size, remain suspended in the air longer, and so are the source of most virus that is transmitted through breathing, as opposed to touching surfaces, where the heavier droplets are likely to accumulate. As I will discuss later, however, very few of the very small aerosols even contain as much as a single virus (Duguid 1945), so it requires a very large number of such small particles to carry an infectious dose. While there remains some disagreement over the relative amount of virus borne by particles of different sizes, most of the virus appears to be associated with particles larger than 1 µ in diameter. This is 500 times the volume of a single coronavirus, and in the size range of particles that are blocked with maximum efficiency by all types of masks. A second point, demonstrated by the studies I just cited, is that N95 masks, and even surgical and cloth masks, can block a large proportion of very small particles or aerosols. What stops these particles is not so much the pore size, but the material or threads that line these pores. The aerosols stick to these threads, a process enhanced, in the case of N95 masks, by electric charges. A common misunderstanding is to view the mask like a sieve, with particles with diameters smaller than that of the pores easily passing through. A better analogy is a fish net. Fish nets frequently trap many fish that are small enough to pass through the mesh of the net, because the fish get tangled up in the cords. This is even more so the case with viruses and masks. Imagine if every time a fish came into contact with the mesh of the net, it was instantly stuck to it. This is basically the way masks work. In fact, masks—including the N95 ones—are typically more efficient at blocking particles that are smaller than the pores, because the smaller the particles, the faster they move around (a process known as Brownian motion), increasing their chances of contacting a thread. The “95” refers to the poorest or least blocking performance—i.e., 95% of particles of some size range are blocked, while 5% penetrate, or pass through. For N95 masks, this range is nominally around 300-500 nm, the pore size. Generally speaking, blocking ability increases for particles smaller, as well as larger, than the pores. Fig. 1 shows the filtration efficiency—that is, % of particles blocked—for an N95 mask surgical mask, and a cloth mask. The exact shape of the curves depends somewhat on the experimental conditions employed, but the concave shape is typical, with a minimum filtration efficiency in this study for particles with diameters in the range of 200-400 nm, and higher efficiencies for both smaller as well as larger particle sizes. 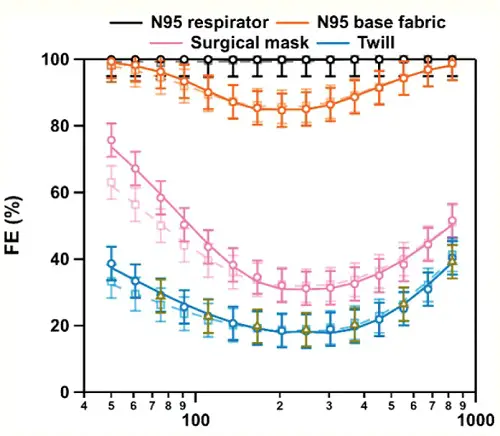
Fig. 1. Particle size vs. filtration efficiency (FE) for an N95 respirator, surgical mask, and a cloth mask.. Particle diameter in nm is shown on the horizontal axis. Note the log scale (from Zangmeister et al. 2020).
There is great variability in the filtration efficiencies for cloth masks, depending on the materials used and the number of layers. Fig. 2 shows several examples: 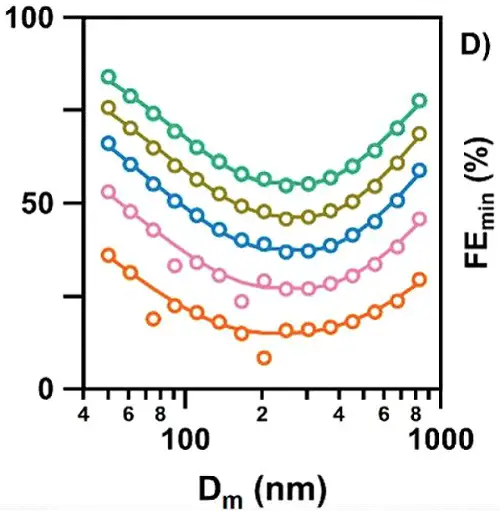
Fig. 2. Effectiveness of different types of cloth masks in blocking aerosol particles of various sizes (from Zangmeister et al. 2020).
Masks of different materials are depicted by different colors, with each circle representing a measurement with particles in a different size range. Notice that most of the masks were at least 50% effective in blocking particles greater than 1000 nm (1 µ) as well as particles smaller than 50 nm. The least efficient blocking occurred with aerosols of intermediate sizes, which are small enough to pass through the pores, but also large enough to move around relatively slowly, reducing the chances of contacting the material lining the pores. The bigger they are, the harder they fallNo mask is perfect; it will not block all aerosols or droplets that we exhale. But even if it blocks only, say, 30-50% of them, this will reduce the concentration of the virus in the air surrounding an infected person, likewise reducing the probability of someone in the vicinity becoming infected. One mathematical analysis performed during the swine flu epidemic estimated that even if masks were only 20% effective, and worn by just 10% of the population, they could significantly reduce the number of infections (Tracht et al 2020). To get a better idea of how effectively masks may reduce transmission of the virus, we need to consider a number of factors that come into play, including: 1) the number of particles someone exhales over time; 2) the number of virions contained in those particles; 3) the length of time the particles remain airborne, and potentially capable of being inhaled by someone in the vicinity; and 4) the number of inhaled virions required to result in infection. Let's consider each of these in turn. Measuring the number and size range of the airborne particles that we exhale is not a simple matter. There are many different technologies and approaches in use, and some may be better able to identify certain sized particles than others. That said, studies have shown that we may exhale as many as thousands, even tens of thousands, of particles per minute (Edwards et al. 2004; Fabian et al. 2007; Chao et al. 2009; Morawska et al. 2009; Stadnytskyi et al. 2020), with the number increasing during loud speaking (Asadi et al. 2019). However, the amount varies considerably among different individuals. Some people, known as high particle producers (HPP), emit as many as thousands of particles per liter of air, with 6-8 liters typically exhaled in a minute. Others, known as low particle producers (LPP), may emit as few as only one particle per liter (Edwards et al. 2004; Fabian et al. 2011). Likewise, a study of virus coughed into the air by flu-infected subjects found that just 4/38 subjects produced 45% of virus collected (Lindsley et al. 2010). Though there is no evidence to date, an obvious speculation is that HPP are more likely to be super-spreaders of the virus, expelling far larger than average quantities of it into the surrounding air. Early studies reported that the great majority, 80-90%, of these particles are very small, less than 1 µ in diameter (Papineni and Rosenthal 1997; Edwards et al. 2004; Morawska et al. 2009; Fabian et al. 2011; Lednicky and Loeb 2013). More recent studies, using light scattering, suggest that there is a much larger fraction of droplets of larger sizes (Stadnytskyi et al. 2020). However, these researchers still reported a mean particle size of about 4 m. Particles like these can remain suspended in the air for a long time (Table 1). 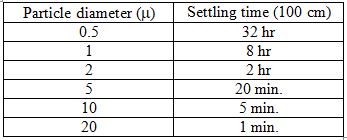
Table 1. Settling times of air-borne aerosols and droplets
These values are very approximate, depending on temperature and relative humidity, and using 100 cm as an arbitrary distance the particle must fall before it's considered to have settled out of the air. As particles fall or settle, they will move progressively outside the breathing range of other people. The assumption is that everyone is standing, with their heads about the same distance above the ground. What is clear, though, is that the smaller the particle, the longer it will remain suspended in the air[1], which means the higher the probability that it will be inhaled by other people. However, there is another relationship, which works in the opposite direction. The smaller the particle, the fewer virions it will contain. I pointed out earlier that aerosols and droplets contain liquid suspensions of virus. Studies have shown that typical concentrations in infected individuals range up to about 106-107 virions per milliliter, or cubic centimeter, with occasional specimens of 100 times greater (To et al. 2020). Given the diameter of an aerosol or droplet particle, it's a simple calculation to determine its volume, and from this value, estimate the number of viruses it may contain. It turns out that the probability that a droplet even as large as 10 µ contains just a single virus is less than 1%, with virtually no chance that it would contain more than one (Stadnytskyi et al. 2020). This figure does have to be adjusted, because within a few seconds or less of when we exhale particles into the air, they're dehydrated, which results in shrinking to roughly 20-30% of their original diameter. So that 10 µ droplet quickly becomes a 2-3 µ aerosol. Still, most particles of this size contain no virus at all, so it takes a very large quantity of them to result in significant exposure. Several studies have reported that 60-80% of airborne virus is associated with particles larger than 1 µ in diameter (Lindsley et al. 2010; Noti et al. 2010; Appert et al. 2012). Stadnytskyi et al. (2020) estimated, based on the rate of emission of all particles, that 1000 virus-containing particles with a median size of 4 µ after dehydration might be exhaled by an infected person during one minute of loud talking. This is consistent with studies of subjects infected with the flu, which have measured virus in the air following breathing, talking or coughing (Lindsley et al. 2010; Yan et al. 2018). From Table 1, we can see that these particles may remain suspended in the air for twenty minutes or more, depending on conditions. So if an infectious person is talking or just breathing for a sustained period of time in the close proximity of other, uninfected individuals, thousands or tens of thousands of virions could become concentrated in the surrounding air. This cloud of viruses represents a serious threat of infection. How serious a threat? This depends on the size of an infectious dose, that is, the number of virions it takes to infect someone. The answer is not known precisely. In theory, a single virus might be sufficient to result in an infection, if it happened to land on a vulnerable cell soon after entering the respiratory tract, and before encountering any immune defense. A single Norwalk virus, which causes gastrointestinal symptoms in humans, has been reported to have a 50% probability of triggering an infection (Teunis et al 2008). However, most viruses have infectious doses ranging from hundreds to thousands of particles (Yezli and Otter 2011). The infectious dose of influenza A is thought to be 2000-3000 virions (Nikitin et al. 2014), while for SARS-CoV, the nearest relative to SARS-Cov-2, it's been reported to be about 300 (Watanabe et al.2010).[2] Based on an infectious dose of 100-1000 virions, Table 2 shows the number of air-borne particles of a certain size necessary to contain that infectious dose, and the approximate length of time they would remain suspended in the air: 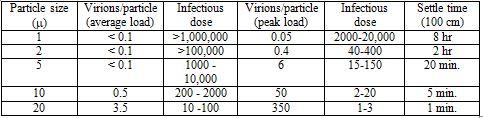
Table 2. Relationship between particle size, infectious dose and settling time
In this Table, I have estimated the number of particles in an infectious dose, based on either an average viral load of 7 x 106 virions/ml., or a peak viral load of 7 x 108 virions per ml. Note that for an average viral load, an infectious dose is unlikely to be carried by particles of less than 1 µ in diameter. While the required number of 1 million or more particles might be exhaled by someone talking for 20 minutes or more, these particles will diffuse over time, reducing their concentration. Also, not all the virions exhaled will necessarily be infectious. Several studies have found that a significant fraction of aerosol-contained viruses are non-infectious (Fabian et al. 2008; Lindsley et al. 2010), and other studies have reported that infective SARS-CoV-2 in the air declines with a half-life of about an hour (van Doremalen et al. (2020). However, for someone with a peak load, an infectious dose could be exhaled into the air within a minute or less. So in many, though not all, situations, even cloth masks, as well as surgical masks and N95 units, should be quite effective in blocking transmission of aerosol particles that are large enough to contain a significant amount of SARS-CoV-2 virus, yet small enough to remain suspended in the air for minutes or longer. Surgical and cloth masks usually don't form a tight seal around the mouth and nose, and for this reason, may not be too effective in protecting the wearer from airborne virus. But even a relatively loosely fitting mask should provide a barrier to virus that is being exhaled by the wearer. It's not a matter of blocking exhalation of all virus, but only enough to reduce the chances of someone's inhaling an infectious dose. If masks are only 50% effective (and many studies report that cloth masks are much better than this), the infectious doses estimated in Table 2 must effectively be doubled; that is, twice as many particles would have to be exhaled for the infectious dose to pass through the mask. We can appreciate that masks will not always prevent an infectious person from spreading the virus, but they should certainly make the difference between doing so and not doing so some of the time. State cases that state the case: the epidemiological evidence for masksGiven the strength of the physical evidence for the effectiveness of masks, one might think that the epidemiological evidence—that is, demonstrations that when large numbers of people in a population wear masks, the case rate for COVID-19 goes down—would be equally robust. It isn't, a point that mask opponents consistently harp on. It has proven quite difficult to obtain consistent evidence of such an effect. However, there are several reasons why we shouldn't expect a clear-cut relationship. To begin, since masks are thought to protect others from the wearer much more effectively than the wearer oneself, relevant studies are difficult to come by. Simply comparing the incidence of infection in people who wear masks vs. those who do not is not likely to show much of an effect—and indeed, this was the basic conclusion of a recent study in Denmark of people wearing masks (Bundgaard et al. 2020). About 2% of the roughly 2500 individuals in each group—masks vs. no masks—tested positive for the virus. While the percentage was slightly lower for the masked group, the difference wasn't significant. We need to compare populations, or large groups of people, who wear masks, with those who don't. There have been several studies of this kind that have concluded that masks significantly decreased virus transmission (Lyu and Wehby 2020; van Dyke et al. 2020; Graves et al. 2020; Karaivanov et al. 2020). In response, however, opponents of wearing facemasks have posted dozens of graphs, from countries all over the world, as well as from individual states and communities in the U.S., showing that case rates continue to rise following mask mandates. When more is lessHow do we reconcile these contrasting findings? Masks, as I emphasized in the earlier discussion of physical evidence, are not a panacea. They won't stop all transmission of the virus, and in some situations, they may not have any effect at all. Still, one would expect that if most people wear them in a large population, they would have a significant effect. One problem, which I also emphasized in an earlier article on lockdowns, is that mask mandates are generally imposed when cases are rising, and lifted when they're falling. So simply producing evidence that case rates are greater during mandates than when no mandate is in effect is not very meaningful.[3] Indeed, since it's very difficult to see how wearing masks could increase transmission of the virus, evidence like this simply confirms the point. However, there are other, subtler fallacies in some of these data. Simply presenting a graph of case rates, and showing that a mask mandate was followed by no change or even a rise in these rates, leaves out important context. To illustrate this, I'm going to examine some of these examples in more detail, taken from a website called rationalground.[4] Here's a graph of the daily case rate in New Mexico: 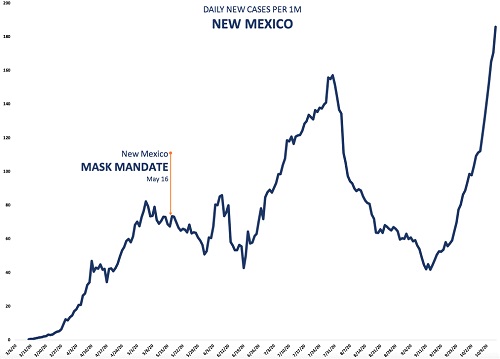
Figure 3. Daily case rate in New Mexico following a mask mandate.[4]
A mask mandate was imposed on May 16. Daily case rates stayed more or less the same for about a month. Then, around the end of June, they shot up, peaking about a month later, before falling. It looks as though the mask mandate didn't help at all. But in the first place, a lockdown in NM, which began on March 24, was to be lifted on May 15, the day before the mask mandate. The mandate was in effect imposed to help alleviate the effect of lifting the lockdown, though the lockdown was later extended to the end of May. But no one is claiming that masks are as effective as lockdowns—certainly, I'm not--so it's not surprising that the case rate did not vary too much for several weeks after the lockdown ended. On the contrary, this suggests that wearing masks probably made the situation better. As I discussed in my article on lockdowns, Part 1 in this series, case rates generally increased in states after a lockdown was lifted. Second, there was widespread opposition to the mandate, with some law enforcement officials refusing to enforce it. It was only when cases began to rise in early July that the state's governor became more aggressive about the mandate, extending it to people exercising, for example. Within several weeks of when this and some other restrictions began, the case rate began to fall dramatically. But most significant, while case rates in NM did rise much of the time during the mask mandate, they rose less than they did in other states that did not have mandates. For example, AZ, a state that borders NM and has a similar climate, had no mask mandate during the period beginning from the middle of May to the end of September, but had more than twice (2.5) the number of cases per millions of people. This suggests that there were likely other factors affecting the case rate, such as the very hot summer weather, which brought many people indoors, where the virus spreads more readily. Both these states, and most others in the southern and western portion of the U.S., had to deal with this problem, and in the case of NM, and other states with a mask mandate, it would greatly complicate a comparison of cases before and after the mandate. To be fair, AZ has a population density more than three times higher than that of NM, and population density is highly correlated with case rate in the U.S.[5] This might account for some of the difference in case rates. However, we might control for the difference in population density to some extent by comparing the total cases from May 16 � Sept 30, when NM's mask mandate was in place, to the total cases in the preceding month, April 16 � May 16. The weather in these states was also cooler at this time. Both states had shelter in place orders during this period, and AZ's total cases per million was actually about 40% lower than NM's. But from the period May 16 � Sept 30, AZ's total cases per million was more than 22 times what it had been in the earlier period, vs. about a 5.5 times increase in NM. So population density can't account for most of the difference in case rates during this later period. Let's further explore the difference between states with and without mask mandates. Here's another example provided by rationalground, Kentucky: 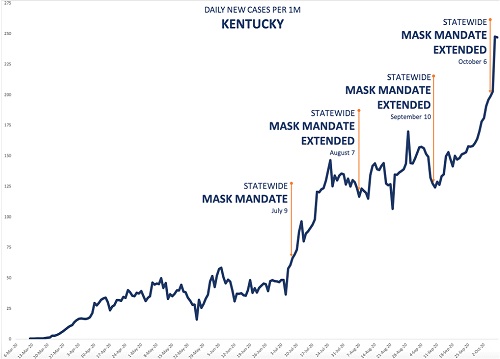
Figure 4. Daily case rate in Kentucky during extended mask mandate.[4]
The state first imposed a mask mandate in early July, and has kept it in force ever since, extending it each time it was due to run out. Yet daily cases continued to increase, except for a period of about a month during August in early September. Again, superficially, it appears that the mask mandate didn't help. But again, suppose we compare KY with a state that had no mandate during this period. In fact, let's compare KY with all the states—there are fifteen of them--that had no such mask mandate. Consider the period from July 9, when KY first mandated facemasks, to Sept 30, when daily case numbers shot up. For the time being, I will cut off the period of interest at that point, because case rates pretty much all over the U.S. rose dramatically at that time, an observation that deserves its own discussion. So just considering this period of about three months, how did the total case rate in KY, with its continuous mask mandate, compare with that in the 15 states that had no state-wide mask mandate at all in this period? The values are shown in Table 3; I have also included NM as another example of a state with a mask mandate. 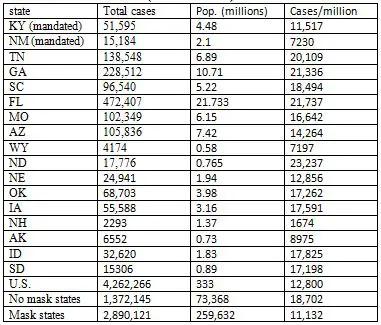
Table 3. Case rates in states without a state-wide mask mandate (7/9/20 � 9/30/30)
Note, first, that KY had a total case rate during this period about half that of Tennessee, a neighboring state with no mask mandate, and a higher population density. In fact, the total cases per million people in KY was less than that of all but three of the fifteen states with no state-wide mask mandate. The exceptions were Alaska, Wyoming and New Hampshire. AK and WY have the lowest population densities of any state in the U.S., while NH (like AK and WY, for that matter) benefited from a cooler summer, and probably less indoor activity. The two states bordering NH, Vermont and Maine, both of which had mask mandates during this period, had lower case rates than NH. Likewise, CO, a mandated state that borders WY, had a lower case rate than its neighbor. In fact, the case rate in KY over this period was almost 40% less than the average case rate in the 15 no-mask states combined (second row from bottom). The rate in the 35 states that did impose state-wide mandates was similar to that of KY (last row). To be fair, in some states where there was no state-wide requirement to wear masks, there were mandates in some cities and other localities. For example, one of the studies I cited earlier as evidence that masks have a significant effect (Graves et al. 2020), compared case rates in counties of TN where masks were required, with rates in counties with no mandate. Such local differences frequently exist within states, making it difficult to say for sure how much the presence or absence of a state-wide mandate affects the actual number of people who wear masks. Even with no requirement anywhere, some if not many people will wear them voluntarily. Mandates also take place in a background in which other social restrictions are usually in place—certain businesses may be closed, for example, and those that are open may have limits to the number of people who are allowed to be together in enclosed areas--and these will have effects on cases as well. Finally, as the preceding discussion illustrates, there are also important contextual factors, such as population density and climate, that can have a significant impact on case rates. But all these additional factors, which generally can't be taken completely into account in any comparison of populations wearing masks with ones that may not be, simply emphasize how oversimplified the arguments of the mask opponents are. The point is that even if one wants to use such simplified data to draw conclusions, we can see in the numbers evidence that supports the conclusion that wearing masks does make a difference. At the very least, we can conclude that these data do not establish that masks have no effect. The opponents of masks haven't proved their case, even if there is no slam-dunk evidence that favors masks. The example of KY applies to many other states used by rationalground to argue against the effectiveness of masks. In state after state, they show that case rates rise following mask mandates. But they generally don't point out that cases rise even faster in states without such mandates. None of their examples include such states, except Florida, where cases rose dramatically over the summer (Table 3). In fact, FL had one of the highest rates of cases of any state in the U.S. over that period, a fact that must be correlated with not only the lack of a mask mandate, but as I discussed in a previous article, a very early end to shelter-in-place, and a more aggressive return to re-opening businesses. The inability to see, or acknowledge, these differences is shown in a tweet by Justin Hart[3], who does show graphs for four states with no mask mandates, and compares them to graphs of three states with mandates. He implies that they're all the same. But they aren't. Three of the four non-mandated states—ND, SD, NE and IA—had a higher rate of total cases during the three month period than any of the three mandated states—MN, WI, and MT. NE, which was slightly lower than WI, has a population density less than one-fourth that of the latter state. The overall average case rate for the three mandated states was 25% lower than that of the four non-mandated states. Cold casesMany of the examples provided by rationalground, particularly of other nations—including France, Spain, Israel, Hungary, and the Philippines—feature daily case rates that are fairly flat for an extended period of time following a mask mandate, and only begin to rise again some time later. Since to the extent that masks work to reduce transmission of the virus, they don't suddenly stop working, this should clue anyone to the notion that other factors are probably coming into play. One of the biggest of these factors is the advent of winter in much of the northern hemisphere. This corresponds with a major surge in cases in France, Hungary, and to some extent Spain, as well as throughout the rest of Europe, and the U.S. As a response to cold weather, people spend more time indoors. Not only does the virus spread more easily in such confined spaces, but most people don't wear masks when inside their own homes, even if they are firmly committed to doing so in public. So one would expect mask mandates to have less effect. This is why earlier I compared case rates in different states up to the end of September. After this point, in the U.S., many states have been affected by the colder weather. Even so, there is evidence that masks can have some effect in these conditions. The two Dakotas, among the most northerly and coldest states in the union, provide a convenient comparison. They lie adjacent, with similar climates and populations. On Nov. 14, the Governor of ND imposed a mask mandate on the state, which was lifted about two months later, on Jan. 18. SD has had no such mandate. In the two and half months preceding the mask mandate, when both states were experiencing a surge in cases, the total cases in the two states were almost identical, 51,056 in ND vs. 50,445 in SD, or an average of 552/day vs. 547/day, though as expressed as a rate per million, ND with a slightly smaller population, had a little bit higher rate. In the two month period of the mandate, the case rate for ND dropped to 509/day, while that for SD rose to 638 per day. The difference was even more dramatic over the final month of the mandate. From Dec.18 � Jan 18, ND recorded 6377 cases, an average of 206/day, while SD recorded 13,191 cases, 426/day. So the case rate in ND was cut by 63% from what it had been prior to the mask mandate, while the rate in SD was cut by about 22%. In fact, we can find lots of neighboring states to compare throughout much of the cold weather surge. Table 4 shows the daily case/million rates in states with and without mask mandates in different regions of the country, where the climate is similar. The period begins on August 1, when most states that were going to order state-wide mandates had done so, up until November 30, by which time the surge had begun, and had come close to peaking in many states. A few states, included ND (as discussed before), NH and IA, imposed mask mandates in November, but they were in effect for only two or three weeks prior to the end of this period: 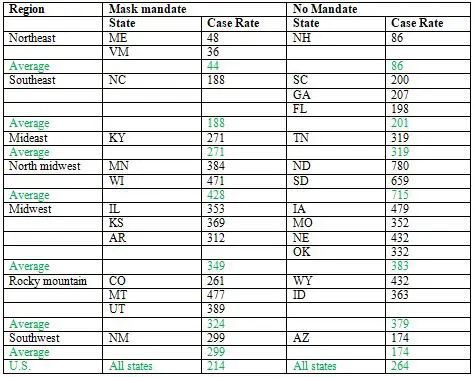
Table 4. Average daily case rates per million in different U.S. regions, 8/1/2020-11/30/2020
In most of the regions, the states with a mask mandate had lower average case rates than states without a mandate in the same region, and the overall average case rate for the region was lower for mandated states than for non-mandated states, as well as for the U.S. as a whole (highlighted in green). The one glaring exception is the southwest. Though we saw earlier that NM, with its state-wide mask mandate, had a much lower case rate that non-mandated AZ up until the end of September, the story has been different for the following two months, and particularly the last two weeks of November, when it recorded about 40% of all the cases occurring in the entire four month period. Cases in AZ have also surged, but somewhat later. For the month of December, the case rate per million in AZ was 822, vs. 703 in NM. The large Native American populations in both states have been hit especially hard, but more so in NM. While AZ has a larger absolute population of this ethnic group, as a % of the total state population, NM's is more than twice as large, almost 10% vs. 4%. Through the end of September, the case rate for Native Americans in NM was more than fifteen times higher than for Caucasians, by far the highest in the U.S.[6] It was about four times higher in AZ. It's relevant to note that the second highest relative case rate, about five, is in Montana, which may account for its relatively high case rate in its region (Table 4).
In most of the regions, the states with a mask mandate had lower average case rates than states without a mandate in the same region, and the overall average case rate for the region was lower for mandated states than for non-mandated states.
A good hair dayA well-known smaller study supporting masks involved two hair stylists working in a salon, known to have COVID-19 symptoms but wearing masks, who served a total of about 140 clients (Hendrix et al. 2020). No cases were reported among any of the clients, nor among other co-workers of the stylists, and all of the clients who were tested (about half of the total), were negative. The stylists served their clients for 5-7 days after developing symptoms, when they should have been infectious. In fact, the stylist who developed symptoms first probably infected the second one, as the two interacted without masks when not serving clients. Moreover, four close contacts of the first stylist, including her husband who she lived with, and two relatives and a third person who lived together in another household, all developed symptoms and tested positive later. This study doesn't prove that masks prevented transmission of the virus, but it's certainly strong evidence. While the hair stylist probably spent more time with the two (at least) individuals that she apparently infected than with any of her clients, the sheer number of the latter would greatly increase the chances of infecting them. If we assume that a client spent an average of 30-60 minutes with one of the hair stylists (and this is probably an underestimate), there was a total of 70-140 hours in which an infected person was in the close presence of another person. This would surely have been more time than the first infected stylist spent unmasked with the second stylist, and probably more time than she spent with her partner during the entire time she was infectious. This study also provides another answer to people who ask why, if masks are effective, their use isn't always associated with a reduction of cases. Both stylists continued to work with clients after developing symptoms. They only stopped work after testing positive. They really should have stopped working as soon as they developed symptoms. The fact that they didn't might have been at least partly because they thought masks were protecting their clients. In this case, it appears that the masks did, but certainly no one is claiming that masks are 100% effective. It may be that when mask mandates are in place, some people engage in riskier behavior than they would otherwise. They may feel that if they have to undergo the inconvenience of wearing a mask, they can at least relax the social distancing a little. Conclusion: there's no coverup to covering upStudies of masks show beyond a shadow of doubt that they are very effective at blocking a large proportion of air-borne particles of the size that carry viruses from an infected person. While opponents of wearing masks claim that a large body of data indicates that they have no effect on case rates in populations, an examination of these studies finds that in fact, many of them are quite supportive of masks. Though mask mandates are not always associated with a reduction in cases, when it's possible to compare these rates with those in similar populations that do not have a mandate, there is very frequently a significant difference. Given the relative inconvenience of wearing masks, I see no justification at all for not doing so. ENDNOTES
REFERENCESAppert J, Raynor PC, Abin M, Chander Y, Guarino H, Goyal SM, Zuo Z, Ge S, Keuhn TH.�(2012)�Influence of suspending liquid, impactor type, and substrate on size-selective sampling of MS2 and adenovirus aerosols.�Aerosol Sci Technol�46:249�257 Ba�azy A, Toivola M, Adhikari A, Sivasubramani SK, Reponen T, Grinshpun SA.(2020) Do N95 respirators provide 95% protection level against airborne viruses, and how adequate are surgical masks? Am J Infect Control 34(2):51-7 Bartoszko JJ, Farooqi MAM, Alhazzani W, Loeb M (2020) Medical masks vs N95 respirators for preventing COVID-19 in healthcare workers: A systematic review and meta-analysis of randomized trials Influenza Other Respir Viruses 14(4):365-373 Bundgaard et al. (2020) Effectiveness of Adding a Mask Recommendation to Other Public Health Measures to Prevent SARS-CoV-2 Infection in Danish Mask Wearers : A Randomized Controlled Trial. Ann Intern Med. Nov 18:M20-6817. Chao CYH, Wan MP, Morawska, L, Johnson GR, Ristovski, ZD, Hargreaves, M, Mengersen, K, Corbett S, Li, Y, Xie X, Katoshevski, D (2020) Characterization of expiration air jets and droplet size distributions immediately at the mouth opening. J Aerosol Sci 40(2):122-133. Duguid, J.P. (1945) The numbers and the sites of origin of the droplets expelled during expiratory activities. Edinb Med J. 52(11):385-401. Edwards DA, Man JC, Brand P, Katstra JP, Sommerer K, Stone HA, Nardell E, Scheuch G. (2004) Inhaling to mitigate exhaled bioaerosols Proc Natl Acad Sci U S A. 101(50):17383�17388 Fabian, P (2008) Influenza Virus in Human Exhaled Breath: An Observational Study PLoS One 3(7):e2691. Fabian, P., Brain, J., Houseman, E.A., Gern, J., Milton, D.K. (2011) Origin of Exhaled Breath Particles from Healthy and Human Rhinovirus-Infected Subjects. J Aerosol Med Pulm Drug Deliv. 24(3):137�147 Graves J et al. (2020) Tennessee Areas Without Mask Requirements Have Higher Death Toll Per Capita https://www.vumc.org/health-policy/news-events/tennessee-areas-without-mask-requirements-have-higher-death-toll-capita Hendrix MJ, Walde C, Findley K, Trotman R. (2020) Absence of apparent transmission of SARS-CoV-2 from two stylists after exposure at a hair salon with a universal face covering policy—Springfield, Missouri, May 2020. MMWR Morb Mortal Wkly Rep 69:930-932 Karaivanov A,�Lu SE,�Shigeoka H,�Chen C,�Stephanie�Pamplona S (2020) Face Masks, Public Policies and Slowing the Spread of COVID-19: Evidence from Canada https://www.medrxiv.org/content/10.1101/2020.09.24.20201178v2 Konda A, Prakash A, Moss GA, Schmoldt M, Grant GD, Guha S (2020) Aerosol filtration efficiency of common fabrics used in respiratory cloth masks. ACS Nano 14(5):6339-6347 Lai ACK, Poon CKM, Cheung ACT (2012) Effectiveness of facemasks to reduce exposure hazards for airborne infections among general populations. J Royal Soc Interface 9(70):938-48 Lednicky, J.A. and Loeb, J.C. (2013)�Detection and isolation of airborne influenza A H3N2 virus using a Sioutas personal cascade impactor sampler.�Influenza Res Treat�2013:656825 Lee S-A, Grinshpun SA, Reponen T (2008) Respiratory performance offered by N95 respirators and surgical masks: Human subject evaluation with NaCl aerosol representing bacterial and viral particle size range. The Annals of Occupational Hygiene 52:177�185. Lindsley WG, Blachere FM, Thewlis RE, Vishnu A, Davis KA, Cao G, Palmer JE, Clark KE, Fisher MA, Khakoo R, Beezhold DH. (2010) Measurements of Airborne Influenza Virus in Aerosol Particles from Human Coughs PLoS One 5(11):e15100. Lindsley WG, Blachere FM, Beezhold DH, Thewlis RE, Noorbakhsh B, Othumpangat S, Goldsmith WT, McMillen CM, Andrew ME, Burrell CN, Noti JD. (2015)�Viable influenza A virus in airborne particles from human coughs.�J Occup Environ Hyg�12:107�113 Lustig SR, Biswakarma JJH, Rana D, Tilford SH, Hu W, Su M, Rosenblatt MS (2020) Effectiveness of common fabrics to block aqueous aerosols of virus-like nanoparticles. ACS Nano 14(6):7651-7658 Lyu W, Wehby GL (2020) Community Use Of Face Masks And COVID-19: Evidence From A Natural Experiment Of State Mandates In The US. Health Affairs 39 (8):1419-1425 Ma Q-X, Shan H, Zhang, H-L, Li, G-M, Yang, R-M, Chen J-M (2020) Potential utilities of mask-wearing and instant hand hygiene for fighting SARS-CoV-2. J Med Virol 92(9):1567-1571 Milton DK, Fabian MP, Cowling BJ, Grantham ML, McDevitt JJ. (2013) Influenza�virus aerosols in�human�exhaled breath: particle size, culturability, and effect of surgical masks. PLoS Pathog. 9(3):e1003205 Morawska, L, Johnson, G, Ristovski Z, Hargreaves M, Mengersen, Kerrie L, Corbett, S, Chao C, Li Y, Katoshevski D (2009) Size distribution and sites of origin of droplets expelled during expiratory activities. Journal of Aerosol Science 40(3):256-69. Nikitin N, Petrova E, Trifonova E, Karpova O. (2014) Influenza Virus Aerosols in the Air and Their Infectiousness. Adv Virol. 2014:859090 Noti JD, Lindsley WG, Blachere FM, Cao G, Kashon ML, Thewlis RE, McMillen CM, King WP, Szalajda JV, Beezhold DH. (2012)�Detection of infectious influenza virus in cough aerosols generated in a simulated patient examination room.�Clin Infect Dis�54:1569�1577 Papineni RS, Rosenthal FS (1997) The size distribution of droplets in the exhaled breath of healthy human subjects J Aerosol Med 10(2)105-16. Rancourt, D (2020) Masks don't work: a review of science relevant to COVID-19 social policy. https://www.rcreader.com/commentary/masks-dont-work-covid-a-review-of-science-relevant-to-covide-19-social-policy Rengasamy S, Eimer B, Shaffer RE. (2010) Simple respiratory protection--evaluation of the filtration performance of cloth masks and common fabric materials against 20-1000 nm�size�particles. Ann Occup Hyg. 54(7):789-98 Shakya KM, Noyes A, Kallin R, Peltier RE. (2017) Evaluating the efficacy of cloth facemasks in reducing particulate matter exposure. J Expo Sci Environ Epidemiol 27(3):352-357 Stadnytskyi, V., Bax, C.E., Bax, A., Anfinrud, P. (2020) The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. Proc Natl Acad Sci U S A. 117(22):11875�11877 To, K. K.-W. et al. (2020) Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect. Dis. 20(5):565-574. Tracht S, Del Valle SY, Hyman JM (2010) Mathematical Modeling of the Effectiveness of Facemasks in Reducing the Spread of Novel Influenza A (H1N1) PLoS One 5(2):e9018 Ueki H, Furusawa Y, Iwatsuki-Horimoto K, Imai M, Kabata H, Nishimura H, Kawakoka Y (2020) Effectiveness of Face Masks in Preventing Airborne Transmission of SARS-CoV-2 mSphere 5(5):e00637-20 Van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, Tamin A, Harcourt JL, Thornburg NJ, Gerber SI, Lloyd-Smith JO, de Wit E, Munster VJ (2020) Aerosol and Surface Stability of�SARS-CoV-2�as Compared with�SARS-CoV-1. N Engl J Med. 382(16):1564-1567. Van Dyke M, Rogers TM, Pevzner E, Satterwhite CL, Shah HB, Beckman WJ,; Farah A, Hunt DC, Rule J (2020) Trends in County-Level COVID-19 Incidence in Counties With and Without a Mask Mandate — Kansas, June 1�August 23, 2020 MMWR Morb Mortal Wkly Rep 69(47):1777-1781 van der Sande M, Teunis P, Sabel R (2008) Professional and Home-Made Face Masks Reduce Exposure to Respiratory Infections among the General Population. PLoS ONE 3(7):e2618. Watanabe T, Bartrand TA, Weir MH, Omura T, Haas CN (2020) Development of a dose-response model for SARS coronavirus. Risk Anal 30(7):1129-38 Whiley H, Keerthirathne TP, Nisar MA, White MAF, Ross KE.Pathogens. 2020 Viral Filtration Efficiency of Fabric�Masks�Compared with Surgical and�N95�Masks. Pathogens 9(9):762 Yan J, Grantham M, Pantelic J, Bueno de Mesquita PJ, Albert Ba, Liu F, Ehrman S, Milton DK (2018) Infectious virus in exhaled breath of symptomatic seasonal influenza cases from a college community Proc Natl Acad Sci U S A. 115(5):1081�1086. Yezli, S., Otter, J.A. (2011) Minimum Infective Dose of the Major Human Respiratory and Enteric Viruses Transmitted Through Food and the Environment. Food Environ Virol�3:1�30 Zangmeister CD, Radney JG, Vincenzi EP, Weaver JL (2020) Filtration efficiencies of nanoscale aerosol by cloth mask materials to slow the spread of SARS-CoV-2. ACS Nano 14(7):9188-9200
|